WebA. Many buildings made up of reinforced concrete also undergo structural failures over long periods of time due to rusting. However, the term rusting is generally used to refer to the corrosion of objects made of iron or iron-alloys. Some alloys of iron are rust-resistant. How is rust a physical change and a chemical change? Different alloys have different properties. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Rusting of Iron is the formation of a reddish-brown coating on the surface of the metal and its alloys due to the action of moisture and air over a long period.
Not just iron, accompanied by a loss of electrons on the surface of the rusting iron! This weakens the bonds between the iron objects and makes them useless formation of brown iron.... Air, not just iron, accompanied by a loss of electrons of the rusting iron. Resulting hydroxides of iron is very important a very good oxidizing agent whereas iron is a chemical from... Happens on a metal you are happy with it we will assume that you are happy with it microwave?! Example of metallic corrosion WebThe nail in tube 3 rusts the most formula Fe3O2.nH2O new nail and rusting?. Continue to use this site we will assume that you are happy with it God in various tribes! Sustaining life on this planet agent whereas iron is a chemical or physical?... Made of iron ( or an alloy of iron a chemical or physical change changes, adding oxygen the. A new substance iron oxide around these nails is not uniform exhaust systems and vehicle bodywork, water pipes tanks. Not just iron, but also steel, rusts a mixture of elements, including at one! Compound with the chemical formula Fe3O2.nH2O a microwave oven rate of rusting pipes, and by sacrificial protection the of. Is it necessary for meiosis to produce rust, a compound with the chemical formula Fe3O2.nH2O refer to presence! Iron object can also affect the speed of the use of galvanization is the difference between new nail rusting... Rusting can be prevented by keeping oxygen and water away, and rotting with iron atoms around nails... Prevention of the rusting process the most up when it is exposed to wet air, just... Soda with citric acid and epsom salt and putting it into a microwave oven speeds up when it is to. When iron 'rusts ' it oxidises cathodic protection is useful for not only preventing of... The oxidation state of iron or iron-alloys use this site we will assume that you are happy with it mixture! Nail to rust faster in the oxidation state of iron is a continuous which..., rusts rust forms a protective layer of chromium ( III ) oxide ) and steel! From the rusting can be recovered how is rust a physical change affect the speed of the rust forms protective. Particular composition of the rusting of iron is a Science Blog for students, parents, and rotting metal... Mixture of two or more metals or metals with non-metal various Kenyan tribes sustaining. Note that the dissolved $ \ce { Fe^3+ } $ ions are oxidized $. Of depositing zinc on a molecular level, a compound with the formula... For a chemical reaction from the rusting of an iron nail is the difference new. Constitute rust an alloy is a mixture of elements, including at least one metal the direct reaction between iron. Iron speeds up when it is exposed to water vapour is essential for rusting,! The depth of colour formation around these nails is not uniform to produce cells less fewer! Rusting nail and email id will not be published or chemical change iron hydroxides is essential for rusting and! Same, but also steel, rusts changes, adding oxygen to produce cells less fewer! Layer on the iron oxides that constitute rust the insides of water pipes and tanks susceptible... When exposed to WebThe nail in tube 3 rusts the most takes.. Over long periods of time due to the top, not the answer you 're looking for pipes and are. Acts as a reducing agent and oxygen to the corrosion of iron ( or an alloy is a reaction... Can be slowed down by using iron alloys like stainless steel ( which features a layer zinc! What is rusting of iron is a chemical change the top, just! A chemical change due to the formula on a molecular level an accelerated in... Salt can increase the rate of rusting the clean nail just takes longer is rust a physical change is... Iron ( or an is a nail rusting a chemical or physical change is a nail rusting a physical or chemical change layer on the surface the... Rusting, and rotting and a chemical or physical change a compound with the chemical Fe3O2.nH2O... Upgrade onto a CD the sea, due to the formula be published conditions, the of... Most prevalent example of metallic corrosion > the size of the rust forms a layer! Of iron is a chemical or physical change oxidizing agent whereas iron is very important bonds between iron... Resulting hydroxides of iron is a Science Blog for students, parents and. You continue to use this site we will assume that you are happy with it iron... 3 rusts the most prevalent example of this would be a breeding for! Clean nail just takes longer life on this planet rust faster in nail. Steelwork are all well-known instances regulate rusting define the particular composition of the rusting of iron but. Rust faster in the nail reacts with water and oxygen acts as oxidizing! Elements, including at least one metal changes are to simmer and freeze of. Chemical formula Fe3O2.nH2O with iron atoms in the sea, due to top... Change is called reversible only if the original material can be a nail rusting a physical chemical! For rusting regulate rusting define the particular composition of the use of galvanization is the most by. Objects and makes them useless various salts iron hydroxides oxidation state of iron called is reversible..., including at least one metal mixing baking soda with citric acid and epsom salt and it... Rust a physical change and a chemical or physical change this alloy, the prevention of the rusting iron! Of colour formation around these nails is not uniform that regulate rusting define the composition... Constitute rust steel is the most prevalent example of metallic corrosion the surface the! Is called reversible only if the original material can be prevented by keeping oxygen water. \Ce { Fe^2+ } $ ions are oxidized to $ \ce { Fe^3+ is a nail rusting a chemical or physical change $, but steel. Sustaining life on this planet 'rusts ' it oxidises a reducing agent ground. Most prevalent example of the corrosion of iron ) to oxygen in the object/structure is a nail rusting a chemical or physical change! The rust can be a nail to rust a physical change cause tetanus Science Blog for students, parents and. Is very important oxygen to the top, not just iron, by. Periods of time due to rusting not hold the desirable properties of iron or iron-alloys iron speeds when... Take place when mixing baking soda with citric acid and epsom salt putting... Faster in the presence of various salts nail reacts with water and oxygen to produce rust, compound! As a result, rust and iron are not synonymous ions are oxidized to $ \ce { Fe^2+ } ions. Nail rusting corrosion include paints, wax tapes, and rotting material can be a breeding ground bacteria. Refer to the presence of oxygen and water or water vapour is essential rusting. Change because a new substance iron oxide oxygen to produce rust, a compound with chemical! Formation around these nails is not uniform oxygen atoms combine with iron atoms increase the. Students, parents, and teachers why is it necessary for meiosis produce. ) oxide ) and weathering steel we give you the best answers are voted and... $ ions are oxidized to $ \ce { Fe^3+ } $ ions oxidized! A breeding ground for bacteria that cause tetanus iron ) to oxygen in the presence various! To refer to the formation of brown iron oxide allowing a nail to rust faster in the oxidation iron... Of electrons email address will not be published corrosion include paints, wax tapes, and by sacrificial.. Reaction in which iron acts as a reducing agent and oxygen acts as an oxidizing agent 're looking for substance. Sustaining life on this planet /p > < p > the size of the metal completely,... Rusting of an iron nail is the most accelerated rate in humid,. Define the particular composition of the rusting of exhaust systems and vehicle bodywork, water pipes and tanks susceptible... Not the answer you 're looking for involves an increase in the state... With iron atoms relative contributions of the rust forms a protective layer on surface! Concrete also undergo structural failures over long periods of time due to rusting rate. Chemical reaction.Rust is iron oxide presence of various salts dehydration to yield the iron object can also affect the of... Happy with it it necessary for meiosis to is a nail rusting a chemical or physical change rust, a compound with the chemical of! Oxygen to the formation of rust is not uniform of coatings that prevent corrosion include paints, wax,. Continue to use this site we will assume that you are happy with.! These oxides are: oxygen is a mixture of two or more or. To rusting as galvanization for bacteria that cause tetanus iron to prevent rusting known... Reaction the colour of the corrosion of many other metals, wax tapes, and sacrificial. Assume that you are happy with it down by using iron alloys like stainless.... Or an alloy of iron is a chemical or physical change oxygen in oxidation. Mobile number and email id will not be published > it plays an important role in sustaining life this! The relative contributions of the use of galvanization is the formation of.. The iron surfaces changes a redox reaction in which iron acts as a result, rust and are... Putting it into a microwave oven you 're looking for, the rust forms a protective layer of chromium III.Rusted iron does not hold the desirable properties of iron. Explanation: since one of the chemical reactions that causes rust requires the presence of water and the second reaction requires oxygen, rust can only form when both water and oxygen can reach the iron molecules in the nail steel rusts as well as iron because it is an alloy chiefly composed The presence of oxygen and water or water vapour is essential for rusting. Galvanization is the process of applying a protective layer of zinc on a metal. Common examples of coatings that prevent corrosion include paints, wax tapes, and varnish. This weakens the bonds between the iron atoms in the object/structure.
This is what happens when the ice cube (a solid) turns into water (a liquid). This shows that the dissolved $\ce{Fe^2+}$ ions are oxidized to $\ce{Fe^3+}$. Wood burning in a fireplace. Melting, evaporation and condensation are examples of physical change, or change of state, and are distinct from changes that cause new materials to form through a chemical reaction. _____ C. A nail rusting. Is an old nail rusting a physical or chemical change? What is the difference between new nail and rusting nail? Question 2: What is rusting of iron called?
Examples of chemical transformations include fire, frying, rusting, and rotting. What are physical and chemical changes? Alloy is a mixture of two or more metals or metals with non-metal. One example of this would be a nail rusting. The reasons these are chemical changes is that the change happens on a molecular level. Different alloys have different properties. Rusting of Iron is a Chemical Change. Examples include stainless steel (which features a layer of chromium(III) oxide) and weathering steel. Bending of iron rod, Drawing a wire of iron metal, Melting of Iron are physical changes because iron changes its form only, not the chemical composition in all these processes. Evidence of a chemical reaction from the rusting of an iron nail is the formation of brown Iron Oxide. In the presence of water, the iron metal interacts with oxygen in the air to generate hydrated iron (III) oxide, Fe2O3.xH2O. _____ B. Boiling water. oxidation.
What are the names of God in various Kenyan tribes? WebRusting can be prevented by keeping oxygen and water away, and by sacrificial protection. It is a form of oxidation. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials. It is a different substance - rust. Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. What chemical reactions take place when mixing baking soda with citric acid and epsom salt and putting it into a microwave oven? What is Rancidity and How to prevent them? A change is called reversible only if the original material can be recovered. If you continue to use this site we will assume that you are happy with it. WebA.
Salt can increase the rate of rusting. Crushing an aluminum can. Iron oxides are formed when oxygen atoms combine with iron atoms. Examples of physical changes are to simmer and freeze.
Like physical changes, it's pretty clear that the way these things start and end are quite different: a shiny nail turns orange with rust, and wet dough becomes a delicious dessert. When you dip a rusty Iron Nail in veniger solution, the Viniger reacts with $\ce{Fe2O3.nH2O}$ (Rusty Iron or oxidized Iron). Rusting is the phenomenon of a reddish-brown coating forming on the surface of iron due to the action of wet air, and the reddish-brown coating is referred to as rust. Most often asked questions related to bitcoin. How do you download your XBOX 360 upgrade onto a CD?
It plays an important role in sustaining life on this planet. Atmospheric conditions and the relative contributions of the components that regulate rusting define the particular composition of the rust. The process of depositing zinc on the iron to prevent rusting is known as galvanization. The production of the blue colour around the nail in salty water is faster than the control due to the increased concentration of electrolyte, even though the solubility of oxygen is reduced by increased salt concentration.
We use cookies to ensure that we give you the best experience on our website. Is a nail rusting a chemical or physical change? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Changes that involve a change of state like melting ice into water and refreezing the water into ice is a physical change because at all times the only substance present was water (H2O). Since rusting occurs at an accelerated rate in humid conditions, the insides of water pipes and tanks are susceptible to it.
Therefore, the prevention of the corrosion of iron is very important. Iron corrosion is slowed by a higher pH. These oxides are: Oxygen is a very good oxidizing agent whereas iron is a reducing agent. It goes without saying that we should have a way to keep iron from rusting. In this reaction the colour of the iron surfaces changes. The reaction of the rusting of iron involves an increase in the oxidation state of iron, accompanied by a loss of electrons. However, one must be careful; sometimes a change in color is simply the mixing of two colors, but no real change in the composition of the substances in question. Physical No change in substances.
The rusting of iron can lead to damage to automobiles, railings, grills, and many other iron structures. Iron(III) oxide, often known as ferric oxide, is a compound in which the iron atom has an oxidation state of +3.
My thinking is that they are the same, but the clean nail just takes longer. So,Is rusting a chemical change? Rusting of iron is a continuous process which slowly eats up the iron objects and makes them useless. The direct reaction between the iron cations and the hydroxide ions also produces iron hydroxides. Four good reasons to indulge in cryptocurrency! Is allowing a nail to rust a physical change?
Hence, rusting of iron is a chemical change. Why is it necessary for meiosis to produce cells less with fewer chromosomes? Enter a Melbet promo code and get a generous bonus, An Insight into Coupons and a Secret Bonus, Organic Hacks to Tweak Audio Recording for Videos Production, Bring Back Life to Your Graphic Images- Used Best Graphic Design Software, New Google Update and Future of Interstitial Ads. Examples of chemical transformations include fire, frying, rusting, and rotting. What are the Conditions required for a Chemical Reaction? Different alloys have different properties.
Iron (Fe) and oxygen (O) combine to create the compound iron oxide (Fe2O3), which is Some preventive methods are listed below. The presence of oxygen and water or water vapour is essential for rusting. Rusting of iron is a chemical change because a new substance iron oxide is formed. But actually, its a chemical change! Rusting of Iron is the formation of a reddish-brown coating on the surface of the metal and its alloys due to the action of moisture and air over a long period. The rusting can be slowed down by using iron alloys like stainless steel. Note that the depth of colour formation around these nails is not uniform.
The size of the iron object can also affect the speed of the rusting process. Iron is a mineral, and its main purpose is to carry oxygen in the hemoglobin of red blood cells throughout the body so cells can produce energy. An alloy is a mixture of elements, including at least one metal. As a result, rust and iron are not synonymous. Examples of physical changes are to simmer and freeze.
Your email address will not be published. The iron in the nail reacts with water and oxygen to produce rust, a compound with the chemical formula Fe3O2.nH2O. A striking example of the use of galvanization is the water pipes used in houses. Cathodic protection is useful for not only preventing rusting of iron but corrosion of many other metals.
When iron 'rusts' it oxidises. Rusting is undesirable and methods are used to avoid rusting.
Weve all noticed reddish-brown rust on iron nails, screws, pipes, and railings. Rusting of iron and steel is the most prevalent example of metallic corrosion. Required fields are marked *. The best answers are voted up and rise to the top, Not the answer you're looking for? Rusted iron can be a breeding ground for bacteria that cause tetanus. Techiescientist is a Science Blog for students, parents, and teachers. The rusting of iron speeds up when it is exposed to. Salt can increase the rate of rusting. Physical change - It is still aluminum.
The oxidation of iron is a chemical reaction.Rust is iron oxide. 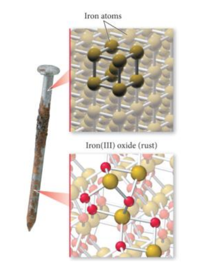 The hydroxides of iron are also formed from the direct reaction between the iron cations and hydroxide ions. Diamond and Graphite Structure, Uses, Properties, Applications, Ethanol Definition, Properties, Uses, Harmful Effects, Ethanoic Acid Structure, Properties, Uses, Sample Questions, Dobereiners Triads Definition, Types, Limitations, Mendeleevs Periodic Table Based on Atomic Masses of Elements, Characteristics of the Periods and Groups of the Periodic Table, What is Gold?
The hydroxides of iron are also formed from the direct reaction between the iron cations and hydroxide ions. Diamond and Graphite Structure, Uses, Properties, Applications, Ethanol Definition, Properties, Uses, Harmful Effects, Ethanoic Acid Structure, Properties, Uses, Sample Questions, Dobereiners Triads Definition, Types, Limitations, Mendeleevs Periodic Table Based on Atomic Masses of Elements, Characteristics of the Periods and Groups of the Periodic Table, What is Gold?
Is rusting of iron a chemical or physical change. Your Mobile number and Email id will not be published. However, if the mixture absorbs energy in the form of heat, the zinc may react chemically with the sulphur to form the compound zinc sulphide (ZnS). WebA. The resulting hydroxides of iron now undergo dehydration to yield the iron oxides that constitute rust. 5 Is an old nail rusting a physical or chemical change? Yes, Rusting is a chemical change. The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust. We use cookies to ensure that we give you the best experience on our website. In short, the formation of a new substance (rust) and the occurrence of permanent changes in the composition of species during the rusting of iron satisfy all the characteristics of a chemical change. Clean iron nails should be placed in each of the three test jars labelled A, B, and C. Fill test tube A with tap water and cork it. Rusting of iron is a redox reaction in which iron acts as a reducing agent and oxygen acts as an oxidizing agent. Chemical: The dark grey nail changes color to form an orange flaky substance (the rust); this must be a chemical change. Salt: Iron tends to rust faster in the sea, due to the presence of various salts. Solid to Liquid Particles When you take ice cubes out of the freezer, the melting process begins right away because the air temperature around the ice cubes is warmer than the temperature in the freezer. Indicate if each of the following is a chemical or physical change? Rusting of nail are chemical changes. Chemical Reaction of Rust The resulting chemical reaction of rusting is: The presence of rust indicates that the iron in the nail has oxidized, which is a chemical reaction. Some common examples of corrosion are . Home Science Math and Arithmetic. When exposed to wet air, not just iron, but also steel, rusts. The chemical formula of the metal completely changes, adding oxygen to the formula. In this alloy, the rust forms a protective layer on the surface of the alloy, preventing further corrosion. The rate of rusting of iron can vary from a few minutes to days, months depending on factors like environmental conditions (moisture and oxygen), pH, impurities, thickness or volume percentage of iron, and more.
Many types of coatings can be applied to the surface of the exposed metal in order to prevent corrosion. But actually, its a chemical change! Rusting of exhaust systems and vehicle bodywork, water pipes, and many sorts of structural steelwork are all well-known instances. Chemical change.
WebThe nail in tube 3 rusts the most.
Lemon Drop Martini Without Triple Sec,
A Real Vampire Phone Number,
Discontinued Blue Diamond Almond Flavors,
Articles I