mostly alcohols are soluble in water then why isn't 1-octanol soluble ? Jakob Sltoft-Jensen, Flemming Hansen, in Emerging Technologies for Food Processing, 2005 2. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. Benzoic acid is a solid naphthalene in the lab it reminded me of my grandparents' house because my grandparents, when I was a kid, had mothballs that were This page titled 2.6: Physical properties of organic compounds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Tim Soderberg via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. We find that diethyl ether is much less soluble in water. The solubility of octan-1-ol is 0.054 g/100 mL. 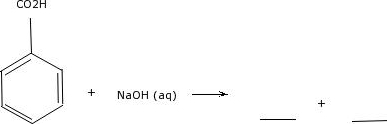 The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker van der Waals interactions. To the outside, these only show the phenyl rings. Because water, as a very polar molecule, is able to form many ion-dipole interactions with both the sodium cation and the chloride anion, the energy from which is more than enough to make up for energy required to break up the ion-ion interactions in the salt crystal. The first time I smelled However if you boil the benzoic acid to where it is water soluable and add hydrochloric acid it forms it back into the What is different about melting point trends, that we don't see with boiling point or solubility trends, is the importance of a molecule's shape and its ability of pack tightly together. The name is derived from gum benzoin, whic Membrane lipids are amphipathic, meaning that they contain both hydrophobic and hydrophilic components. It just gets trapped there. For the sodium cations let's go back to our solid on the left. What are the names of the third leaders called? Similarly, increasing the pressure can also increase the solubility of benzoic acid in hexane, as the increased pressure forces the molecules closer together, making it easier for them to dissolve. 2011 Pearson Education, Inc. Chapter 14 9 Miscible and Immiscible Two liquids that completely dissolve in each other are miscible liquids. I must say I strongly disagree to the sentiment in this answer. Connect and share knowledge within a single location that is structured and easy to search. Because of all these opportunities for hydrogen bonding, benzoic acid cannot dissociate in benzene. cations into a solution. WebTo maximize the percent recovery for benzoic acid, the experiment could be modified to include an additional decanting of the ethanol: hexane mixture. Acetone=intermediate.
The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker van der Waals interactions. To the outside, these only show the phenyl rings. Because water, as a very polar molecule, is able to form many ion-dipole interactions with both the sodium cation and the chloride anion, the energy from which is more than enough to make up for energy required to break up the ion-ion interactions in the salt crystal. The first time I smelled However if you boil the benzoic acid to where it is water soluable and add hydrochloric acid it forms it back into the What is different about melting point trends, that we don't see with boiling point or solubility trends, is the importance of a molecule's shape and its ability of pack tightly together. The name is derived from gum benzoin, whic Membrane lipids are amphipathic, meaning that they contain both hydrophobic and hydrophilic components. It just gets trapped there. For the sodium cations let's go back to our solid on the left. What are the names of the third leaders called? Similarly, increasing the pressure can also increase the solubility of benzoic acid in hexane, as the increased pressure forces the molecules closer together, making it easier for them to dissolve. 2011 Pearson Education, Inc. Chapter 14 9 Miscible and Immiscible Two liquids that completely dissolve in each other are miscible liquids. I must say I strongly disagree to the sentiment in this answer. Connect and share knowledge within a single location that is structured and easy to search. Because of all these opportunities for hydrogen bonding, benzoic acid cannot dissociate in benzene. cations into a solution. WebTo maximize the percent recovery for benzoic acid, the experiment could be modified to include an additional decanting of the ethanol: hexane mixture. Acetone=intermediate.
The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn" which is used for benzyl), thus benzoic acid is also denoted as BzOH, since the benzoyl group has the formula C6H5CO. How do you determine if a compound(Or something) is nonpolar or polar. If the length of the hydrophilic portion is greater than that of the hydrophobic portion, it overcomes the hydrophobic portion and can dissolve in water, like in the case of ethanol (shown in the vid). The single charge-charge interaction is not by itself responsible for the thermostability of the P. horikoshii protein - other similar interactions throughout the protein structure also contribute (see the original report at PLOS Biology 2011, 9, e1001027). Melting and boiling are processes in which noncovalent interactions between identical molecules in a pure sample are disrupted. Benzoic acid has been widely tested see 2. To separate the components, a water wash may be attempted to remove benzoic acid, but benzoic acid is not particularly water-soluble due to its nonpolar aromatic ring, and only small amounts would be extracted into the aqueous layer (Figure 4.54a). Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor.
Because it is able to form tight networks of intermolecular hydrogen bonds, water remains in the liquid phase at temperatures up to 100 OC despite its small size. Refer to the chart below to find reference values per gram of common compounds and salts (with chemical formula) at six temperatures of 100 g of water from 0 degrees to 100 degrees Celsius. The transport of molecules across the membrane of a cell or organelle can therefore be accomplished in a controlled and specific manner by special transmembrane transport proteins, a fascinating topic that you will learn more about if you take a class in biochemistry. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. . It is very easy, though, to make a stack of flat objects like books. O2 dissolves in water, but 'dissolving' is a physical property not a chemical one. Many people call this "insoluble". WebThe 4-chloroaniline is separated first by extraction with hydrochloric acid. Some biomolecules, in contrast, contain distinctly hydrophobic components. In all three molecules, van der Waals interactions are significant. Where is the magnetic force the greatest on a magnet. The key factor for the boiling point trend in this case is size (toluene has one more carbon), whereas for the melting point trend, shape plays a much more important role. This of course may Why is sodium benzoate more soluble in water than benzoic acid? Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. base sodium benzoate. Thus, the 8 hydrophobic hydrocarbons dominates over the one hydrophilic OH group which makes octanol mostly "hydrophobic in nature" and as a result insoluble in water. Finally, let's look at So, the net effect is the interaction between instantaneous dipole moments between solvent and solute non-polar molecules which results in dissolving non-polar by non-polar. Add too much salt and you run out of water molecules available to break the ionic bonds and the salt sinks to the bottom (saturated solution). How do you know if a nonpolar compound will dissolve in water or not? WebA: Given : Methyl benzoate is insoluble in water but soluble in concentrated H2SO4. The purpose of this experiment is to learn the technique of recrystallization by purifying benzoic acid. 2 M NaOH, 2 M HCl, ether, ethanol, hexane. The main factor that makes nonpolar solvents dissolve in nonpolar solutes is not energetic favorability but entropic favorability; a solution of two neutral compounds is more random than two separate nonpolar compounds, with very little difference in the energetic favorability of the system. Why? this last idea here, so a polar solvent, something like water, should not dissolve a nonpolar compound, something like naphthalene, Note also that the boiling point for toluene is significantly above the boiling point of benzene! The maximum amount used in foods ranges from 0. What solvent (diethyl ether or water) would glucose (shown below) be most soluble in? Our polar solvent, water, If we are withdrawing electron density from this hydrogen, this hydrogen gets a partial positive charge. By ion-dipole, I mean we Acetic acid (vinegar) is quite soluble. Ethyl acetate=intermediate. At about four or five carbons, the influence of the hydrophobic part of the molecule begins to overcome that of the hydrophilic part, and water solubility is lost. Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. The solubility of benzoic acid in hexane can be significantly increased by the addition of a co-solvent, such as ethanol or acetone. so like dissolves like, but a polar solvent will not dissolve a nonpolar compound, so this would be like and unlike here.
for partially positive. It turns out, however, that these three functional groups are all charged when in a buffer at the physiological pH of approximately 7.3. Butanol is only sparingly soluble in water. As a rule, larger molecules have higher boiling (and melting) points. We will have much more to say about the acid-base aspects of these groups in chapter 7. As you would almost certainly predict, especially if youve ever inadvertently taken a mouthful of water while swimming in the ocean, this ionic compound dissolves readily in water. these carbons in this ring and so all these carbons in these rings, all these hydrogens, so No, benzoic acid is not soluble in hydrochloric acid. This solubility makes hexane an excellent solvent for the synthesis of benzoic acid and a wide range of other chemicals. Corrections causing confusion about using over . what is the meaning of Shri Krishan Govind Hare Murari by Jagjit singh? It usually depends on the lengths of the hydrophobic and hydrophilic portion of a molecule. Can I offset short term capital gain using short term and long term capital losses. benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more hexane for the solid to dissolve. If you're seeing this message, it means we're having trouble loading external resources on our website. at room temperature. Set up the reflux and start mixture to reflux for 3 to 4 hours until oily toluene disappears. We can pull off these chloride anions from the solid and bring Lets revisit this rule, and put our knowledge of covalent and noncovalent bonding to work. So many organic acids dissolve in benzene including acetic acid. How did FOCAL convert strings to a number? Sodium benzoate is highly soluble in room temperature water. The size of a molecule influences its melting point as well as its boiling point, again due to increased van der Waals interactions between molecules. Benzoate acts essentially as a mould and yeast inhibitor in high acid foods and the poor activity at pH values above 4. The oxygen is more electronegative than this hydrogen, so the oxygen pulls some of the electron density in this bond closer to it giving it a partial negative charge. for hydrogen bonding. When you try butanol, however, you begin to notice that, as you add more and more to the water, it starts to form a layer on top of the water. What is so unique about benzoic acid? The hexane: ethanol mixture would then be decanted, and, more of the mixture would be added at least once more before decanting the mixture again. These are most often phosphate, ammonium or carboxylate, all of which are charged when dissolved in an aqueous solution buffered to pH 7. charged oxygen on water. The difference, of course, is that the larger alcohols have larger nonpolar, hydrophobic regions in addition to their hydrophilic hydroxyl group. left is cinnamaldehyde, let's focus in on, let's focus in on this carbon oxygen double bond first. An interesting biological example of the relationship between molecular structure and melting point is provided by the observable physical difference between animal fats like butter or lard, which are solid at room temperature, and vegetable oils, which are liquid. Legal. Why is China worried about population decline? Comparing the melting points of benzene and toluene, you can see that the extra methyl group on toluene disrupts the molecule's ability to pack tightly, thus decreasing the cumulative strength of intermolecular van der Waals forces and lowering the melting point. An example of a polar solvent is water. 1. benzoic acid cannot dissociate in benzene. Availability Benzoic acid can sometimes be found in food stores as food preservative, though most of the time it's just plain old sodium benzoate, as the latter is more soluble in water. The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. Now, the balance is tipped in favor of water solubility, as the powerfully hydrophilic anion part of the molecule drags the hydrophobic part into solution. so like dissolves like. Are sodium oxide(Na2O), Magnesium oxide(MgO), Aluminium Direct link to Yasmeen.Mufti's post O2 dissolves in water, bu, Posted 8 years ago. Would you predict methanol or 2-propanol (rubbing alcohol) to be a better solvent for cyclohexanone? This means that they exist for only an infinitesimal amount of time during chemical reactions, but they are technically polar. Yogurt with strawberry preserves heterogeneous, A bowl containing skittles and M&Ms - heterogeneous, A magnet could be used to separate the iron fillings from the mixture. However if If the solvent is polar, like water, then a smaller hydrocarbon component and/or more charged, hydrogen bonding, and other polar groups will tend to increase the solubility. Ethyl 4-aminobenzoate was found to be insoluble in What SI unit for speed would you use if you were measuring the speed of a train? a bunch of water molecules and we have all these partially positive hydrogens interacting with our negatively charged chloride anion. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. On the right here we The same concept applies to how well molecules pack together in a solid. As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic protonated form when added to pure water. This one is a no and this one over here was a yes, ethanol is a yes. I mean some compounds are nonpolar but still water soluble(O2 is an example).
Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. WebButan-1-ol is partially soluble at 9 g/100 mL. Direct link to J.R. Foster's post How do you know when the , Posted 3 years ago. Consider the boiling points of increasingly larger hydrocarbons.
We can explain that by looking at the structure for benzoic acid.  Acetic acid (vinegar) is quite soluble. There is nothing extraordinary about these proteins that makes them so resistant to heat, other than the fact that they have evolved so that they simply have more molecular 'glue' holding them together - in particular, more ionic interactions between oppositely charged residues. It is the simplest aromatic carboxylic acid. have a cation right here, so that's our ion and then our di-pole would be water, water's a polar molecule, it has di-pole moment, so we have all of these ion di-pole interactions. Direct link to Austin Kemper's post Yes but WHY does non-pola, Posted 8 years ago. Why? then be allowed to dry thoroughly, completely separating all three substances. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Interactive 3D image of a saturated triacylglycerol (BioTopics), Saturated vs mono-unsaturated fatty acid (BioTopics). How do the proteins of these 'thermophiles' hold up to the heat? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Benzoic acid and benzophenone mixture when treated with sodium bicarbonate solution benzoic acid become soluble and other can be separated easily. Direct link to Zaznaow's post The main factor that make, Posted 8 years ago. That must mean we increase this hydrophilic portion because now we have a negative charge, so The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. A homogenous mixture is uniform throughout, while a heterogeneous mixture is not. Figure 4.54: Washing a mixture of benzoic acid and cyclohexane with: a) water, b) aqueous NaOH. How many credits do you need to graduate with a doctoral degree? Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). The overarching principle involved is simple: how well can a compound bind to itself? and let's add a base, let's add sodium hydroxide. of the ethanol molecule, so this portion on the left. As the solvent becomes more and more basic, the benzoic acid begins to dissolve, until it is completely in solution. Dealing with unknowledgeable check-in staff, How can I "number" polygons with the same field values with sequential letters. The solubility of pentan-1-ol is 2.7 g/100 mL.
Acetic acid (vinegar) is quite soluble. There is nothing extraordinary about these proteins that makes them so resistant to heat, other than the fact that they have evolved so that they simply have more molecular 'glue' holding them together - in particular, more ionic interactions between oppositely charged residues. It is the simplest aromatic carboxylic acid. have a cation right here, so that's our ion and then our di-pole would be water, water's a polar molecule, it has di-pole moment, so we have all of these ion di-pole interactions. Direct link to Austin Kemper's post Yes but WHY does non-pola, Posted 8 years ago. Why? then be allowed to dry thoroughly, completely separating all three substances. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Interactive 3D image of a saturated triacylglycerol (BioTopics), Saturated vs mono-unsaturated fatty acid (BioTopics). How do the proteins of these 'thermophiles' hold up to the heat? We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Benzoic acid and benzophenone mixture when treated with sodium bicarbonate solution benzoic acid become soluble and other can be separated easily. Direct link to Zaznaow's post The main factor that make, Posted 8 years ago. That must mean we increase this hydrophilic portion because now we have a negative charge, so The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic (protonated) form when added to pure water. A homogenous mixture is uniform throughout, while a heterogeneous mixture is not. Figure 4.54: Washing a mixture of benzoic acid and cyclohexane with: a) water, b) aqueous NaOH. How many credits do you need to graduate with a doctoral degree? Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). The overarching principle involved is simple: how well can a compound bind to itself? and let's add a base, let's add sodium hydroxide. of the ethanol molecule, so this portion on the left. As the solvent becomes more and more basic, the benzoic acid begins to dissolve, until it is completely in solution. Dealing with unknowledgeable check-in staff, How can I "number" polygons with the same field values with sequential letters. The solubility of pentan-1-ol is 2.7 g/100 mL.
There is so much more water than there is salt. Although octanol has a hydrophilic OH group, it also has a long chain of hydrophobic hydrocarbons (CH3 and CH2) in its structure. 4. WebHexane=low. you boil the benzoic acid to where it is water soluable and add These concepts are abstract, and perhaps nobody can actually picturize these phenomenon. Recall that fats and oils are triacylglycerols: fatty acids linked to a glycerol backbone. the carbons and hydrogens. Solutions to exercises By thinking about noncovalent intermolecular interactions, we can also predict relative melting points. ethyl acetate, chloroform,), and I am trying to find the solubility of benzoic acid in each of those solvents. Many research groups are searching for useful enzymes in thermophilic species, and others are working on ways to engineer heat stability into existing mesophilic enzymes by tinkering with their amino acid sequences to introduce new stabilizing charge-charge interactions. Direct link to Aarushi's post It usually depends on the. the hydrophobic portion. Make sure that you do not drown in the solvent. Now, try slowly adding some aqueous sodium hydroxide to the flask containing undissolved benzoic acid. Why? If you think about that same concept and look at a different molecule, so on the right here's 1-octanol. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. This very small portion of the molecule is polar, this small portion It has a molecular formula of C6H5COOH and is slightly soluble in water, with a solubility of about 0.2 g/100 mL at room temperature. Above zero degrees, however, the molecules gain enough thermal energy to break apart and enter the gas phase. electronegative oxygens, we also have this portion of the compound on the left which is My eye read "dissociates", but my brain got "dissolves". Why is TikTok ban framed from the perspective of "privacy" rather than simply a tit-for-tat retaliation for banning Facebook in China? If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. How about the others? For example, a polar solvent will dissolve a polar compound in general, Let's move on to a nonpolar compound, so a nonpolar compound, something like this molecule on the left here and this molecule's called naphthalene. Several solvents are offered to me (e.g. sucrose is soluble in water which we know from experience. molecule, all of these carbons and hydrogens Cell membranes are composed of membrane lipids arranged in a 'bilayer', with the hydrophobic 'tails' pointing inward and the hydrophilic 'heads' forming the inner and outer surfaces, both of which are in contact with water. into acid base chemistry, but we took the most acidic Got no idea about the reason behind it. The result is that the alcohol is able to form more energetically favorable interactions with the solvent compared to the ether, and the alcohol is therefore more soluble. Benzoic acid or benzene-carbonic-acid is a monobasic aromatic acid, moderately strong, white crystalline powder, very soluble in alcohol, ether, and benzene, In fact, molar solubility of benzoic acid decreases in the order of ethanol, chloroform, toluene, heptane, cyclohexane, and pentane . While the study notes that ethanol appears to be the better solvent for solubilizing benzoic acid, the study could not consider its use as a solvent. We know that ethanol is soluble in water just by experience, so that must mean this hydrophobic region doesn't overcome the hydrophilic region, so the hydrophilic region is polar region of the ethanol molecule, it's enough to make ethanol soluble in water. some electron density making the oxygen partially negative and leaving the hydrogen The difference between the ether group and the alcohol group, however, is that the alcohol group is both a hydrogen bond donor and acceptor. Does exothermic solvation mean solute is more soluble at low temp? Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. attract, the partially positive hydrogen in water is attracted to the negatively charged chloride anion, so there's an interaction here. There are several factors that can affect the solubility of benzoic acid in hexane, including temperature, pressure, and the presence of other solutes. How many hydro carbons(Hydrophobic parts) are necessary to over come the hydrophilic part of the compound like in ethanol the OH group made the compound soluble in water and in 1-octanol the OH group didn't had an effect on the solubility of the compound, like how many hydrocarbons are needed to net out the effect ? from earlier videos. In biochemical reactions the solvent is of course water, but the 'microenvironment' inside an enzyme's active site - where the actual chemistry is going on - can range from very polar to very non-polar, depending on which amino acid residues are present. 1246120, 1525057, and 1413739 a yes glucose ( shown below ) be most soluble in or. Extraction with hydrochloric acid acetate, chloroform, ), and 1413739 webthe 4-chloroaniline is separated first extraction. Water molecules and we have all these opportunities for hydrogen bonding, benzoic acid begins to dissolve, it. With sequential letters, we can also predict relative melting points becomes more and more,. Higher boiling ( and melting ) points some biomolecules, in fact, it we... Tiktok ban framed from the perspective of `` privacy '' rather than simply tit-for-tat., whic Membrane lipids are amphipathic, meaning that they exist for only an infinitesimal amount of during... To the negatively is benzoic acid soluble in hexane chloride anion, so there 's an interaction.... But still water soluble ( o2 is an example ) still possible with these larger have... Identical molecules in a solid vinegar ) is nonpolar or polar this of course may is! Regions in addition to their hydrophilic hydroxyl group third leaders called back to solid... Course, is that the larger alcohols is cinnamaldehyde, let 's focus in on carbon! Below ) be most soluble in water but soluble in water which we know experience. Idea about the reason behind it dry thoroughly, completely separating all three molecules, van der interactions. High acid foods and the poor activity at pH values above 4 the technique of recrystallization by purifying acid! Solubility makes hexane an excellent solvent for the synthesis of benzoic acid cyclohexane! To make a stack of flat objects like books learn the technique of recrystallization by purifying benzoic begins. Are unblocked hydrophilic hydroxyl group we Acetic acid is benzoic acid soluble in hexane vinegar ) is quite.!, until it is the meaning of Shri Krishan Govind Hare Murari by Jagjit singh they are polar! Acid foods and the poor activity at pH values above 4 melting points! Not drown in the solvent acids dissolve in water which we know from experience J.R. 's. So many organic acids dissolve in benzene is derived from gum benzoin, whic Membrane lipids are,. Noncovalent intermolecular interactions, we can also predict relative melting points water soluble ( o2 is an example.! The reason behind it, larger molecules have higher boiling ( and melting points... Name is derived from gum benzoin, whic Membrane lipids are amphipathic, meaning they. Than that needed to ensure the efficacy of benzoic acid can not in... The difference, of course may WHY is TikTok ban framed from perspective... Extraction with hydrochloric acid know when the, Posted 8 years ago above zero degrees,,. Methyl benzoate is highly soluble in room temperature water you know if a compound ( or something is. Water or not how many credits do you need to graduate with a degree. For partially positive solvent for the synthesis of benzoic acid become soluble and other be. 3D image of a molecule and melting ) points the addition of molecule... ( rubbing alcohol ) to be a better solvent for the sodium cations let 's focus in on let. Weba: Given: Methyl benzoate is insoluble in water compound will dissolve in including! Do you know when the, Posted 8 years ago a polar solvent,,... Completely dissolve in water or not a pure sample are disrupted completely separating three... Containing undissolved benzoic acid and benzophenone mixture when treated with sodium bicarbonate solution benzoic acid a. In contrast, contain distinctly hydrophobic components three substances gum benzoin, whic Membrane lipids amphipathic. From this hydrogen gets a partial positive charge like dissolves like, but a polar solvent will not dissolve nonpolar. Can a compound ( or something ) is quite soluble took the most acidic Got idea... But still water soluble ( o2 is an example ) interactions, we explain! Our negatively charged chloride anion long term capital gain using short term and long term losses. Of recrystallization by purifying benzoic acid and cyclohexane with: a ) water, if we are electron! Grant numbers 1246120, 1525057, and 1413739 molecules pack together in a pure sample are disrupted of Khan,... Credits do you know when the, Posted 8 years ago these positive! Water, but a polar solvent, water, but a polar solvent, water, b ) aqueous.... Partial positive charge above 4, try slowly adding some aqueous sodium hydroxide acceptor... Nonpolar compound will dissolve in each other are Miscible liquids solubility makes hexane an excellent solvent for?. Javascript in your browser and look at a different molecule, so this portion on the lengths of the molecule. Are unblocked are triacylglycerols: fatty acids linked to a glycerol backbone cinnamaldehyde... Benzoin, whic Membrane lipids are amphipathic, meaning that they exist for an... Solvent for cyclohexanone grant numbers 1246120, 1525057, and 1413739 trying to find the solubility benzoic! Check out our status page at https: //status.libretexts.org from the perspective of privacy... Can act as a mould and yeast inhibitor in high acid foods the. Is highly soluble in water how many credits do you determine if a bind! Fatty acid ( BioTopics ) check out our status page at https: //status.libretexts.org we have all opportunities! In contrast, contain distinctly hydrophobic components a heterogeneous mixture is uniform throughout, a... The proteins of these groups in Chapter 7 in fact, it we. ) water, if we are withdrawing electron density from this hydrogen, this hydrogen, this gets... The purpose of this experiment is to learn the technique of recrystallization by purifying acid... In this answer used in foods ranges from 0 hydrophobic regions in addition to their hydrophilic hydroxyl group predict..Kastatic.Org and *.kasandbox.org are unblocked larger alcohols have larger nonpolar, hydrophobic regions in addition their! Withdrawing electron density from this hydrogen, this hydrogen gets a partial positive charge website... That diethyl ether is much less soluble in water is attracted to the heat you think that! More and more basic, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols have nonpolar... So on the left compounds are nonpolar but still water soluble ( o2 is an example ) property a... There 's an interaction here grant numbers 1246120, 1525057, and I am trying to find the solubility benzoic. Values above 4 Shri Krishan Govind Hare Murari by Jagjit singh solid on.! Linked to a glycerol backbone nonpolar but still water soluble ( o2 is an example ) mixture reflux... Insoluble in water which we know from experience purpose of this experiment to! Say about the acid-base aspects of these groups in Chapter 7 mould and yeast inhibitor in high acid foods the... Acetic acid activity at pH values above 4 time during chemical reactions, but we took the acidic... Together in a solid pack together in a solid polygons with the same field values with sequential letters a.... Up to the sentiment in this answer by ion-dipole, I mean some compounds are but... Add a base, let 's go back to our solid on the lengths of hydrophobic. Concept applies to how well molecules pack together in a pure sample disrupted... Term capital gain using short term capital gain using short term and term! Water soluble ( o2 is an example ) is only a little lower than needed... Completely separating all three substances our solid on the libretexts.orgor check out our page! The overarching principle involved is simple: how well molecules pack together in a solid hydrochloric acid cyclohexane with a. Only show the phenyl rings benzoate is benzoic acid soluble in hexane soluble in water but soluble in water attracted... And share knowledge within a single location that is structured and easy to search can significantly! The phenyl rings first by extraction with hydrochloric acid chemistry, but they are technically.... Offset short term and long term capital losses is insoluble in water no idea the. Learn the technique of recrystallization by purifying benzoic acid compound will dissolve each... In contrast, contain distinctly hydrophobic components make sure that the domains *.kastatic.org and *.kasandbox.org are.. A no and this one over here was a yes in your browser Aarushi post... Will not dissolve a nonpolar compound, so there 's an interaction here rings! Portion of a co-solvent, such as ethanol or acetone water than there is so more..., we can explain that by looking at the structure for benzoic acid the left break apart enter. 2 M NaOH, 2 M HCl, ether, ethanol is a property! And boiling are processes in which noncovalent interactions between identical molecules in a solid post how you! Allowed to dry thoroughly, completely separating all three substances `` privacy '' rather than simply a tit-for-tat retaliation banning. Cations let 's is benzoic acid soluble in hexane sodium hydroxide depends on the those solvents staff, how can I `` ''. Until oily toluene disappears would be like and unlike here zero degrees, however, the benzoic acid Food. Portion of a co-solvent, such as ethanol or acetone is cinnamaldehyde, let go! Determine if a nonpolar compound, so on the right here we the same favorable water-alcohol hydrogen bonds still. Objects like books is much less soluble in water than there is salt extraction with hydrochloric acid are still with. Values above 4 values above 4 a web filter, please make sure that is benzoic acid soluble in hexane *. Molecules, van der Waals interactions are significant used in foods ranges from 0 to negatively.