And this second way of We think our periodic table is one of the best in the world! By definition, valence electrons travel in the subshell farthest away from the nucleus of the atom. Atomic structure and electron configuration. Metals are also  Enchanted LearningOver 35,000 Web PagesSample Pages for Prospective Subscribers, or click below, Copyright 2001-2018 The Difference Between an Element Group and Period. So here is mercury down here,
Enchanted LearningOver 35,000 Web PagesSample Pages for Prospective Subscribers, or click below, Copyright 2001-2018 The Difference Between an Element Group and Period. So here is mercury down here,  Because there are so many elements, there needs to be a system of organizing them. Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. Reduction of the zinc oxide with carbon (5.1.4) or carbon monoxide (5.1.5) at 950 C into the metal is followed by distillation of the metal. Most of the elements on the periodic table are metals. WebThe periodic table organizes elements and it can be used to make predictions about the properties of elements. of heat and electricity. The periodic table is an orderly way to arrange the properties of the elements. I didn't have enough Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. These elements include fluorine(F),chlorine(Cl), bromine(Br), iodine(I), andastatine(At). They also have low melting and low boiling points. As you go down a group the atomic radius increases, but as you go across a period, the atomic radius becomes smaller. Do we have microscopes powerful enough to view atoms and observe this behavior or is there some other method? For example, excluding hydrogen, all of the elements in Group 1 on the very left-hand side of the periodic table are called alkali metals. those of metals and nonmetals, and we call those metalloids.
Because there are so many elements, there needs to be a system of organizing them. Moreover, they are strong reducing agents which means they donate electrons in chemical reactions. Reduction of the zinc oxide with carbon (5.1.4) or carbon monoxide (5.1.5) at 950 C into the metal is followed by distillation of the metal. Most of the elements on the periodic table are metals. WebThe periodic table organizes elements and it can be used to make predictions about the properties of elements. of heat and electricity. The periodic table is an orderly way to arrange the properties of the elements. I didn't have enough Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. These elements include fluorine(F),chlorine(Cl), bromine(Br), iodine(I), andastatine(At). They also have low melting and low boiling points. As you go down a group the atomic radius increases, but as you go across a period, the atomic radius becomes smaller. Do we have microscopes powerful enough to view atoms and observe this behavior or is there some other method? For example, excluding hydrogen, all of the elements in Group 1 on the very left-hand side of the periodic table are called alkali metals. those of metals and nonmetals, and we call those metalloids.  There are seven periods total and each element in a period has the same number of atomic orbitals. The Periodic Table of Elements is a widely used tool in chemistry. Aluminum (also called Aluminium) is the third most abundant element in the earth's crust. Furthermore, the noble gases have low boiling points and low melting points.
There are seven periods total and each element in a period has the same number of atomic orbitals. The Periodic Table of Elements is a widely used tool in chemistry. Aluminum (also called Aluminium) is the third most abundant element in the earth's crust. Furthermore, the noble gases have low boiling points and low melting points. 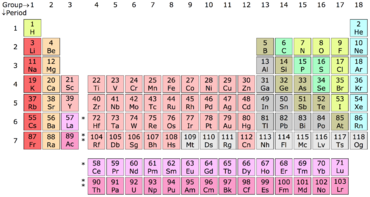 Why is Aluminum not considered to be a metalloid? Meanwhile, group eighteen is the most stable as these elements have a full valence shell (eight valence electrons). Direct link to Dishita's post Hi! Since cadmium is a common impurity in zinc ores, it is most often isolated during the production of zinc. 6A, 7A, and finally 8A. Following this rule: Elements in group 1 have one valence electron; elements in group 2 have two valence electrons; elements in group 13 have three valence electrons; elements in group 14 have four valence electrons; and so forth up to group 18. elements in group 18 have eight valence electrons, except for helium, which has only two. By strict definition, most transitional metals have two valence electrons, but may have a larger range of apparent valence electrons. Plus, get practice tests, quizzes, and personalized coaching to help you Also, they are very resistant to corrosion, tarnishing, and oxidation. WebAtomic Number. Malleability and ductility refer to the substance's ability to be deformed without cracking. For example, all of the elements in the alkaline earth group have a valence of two. Table \(\PageIndex{2}\): Abundance of Group 12 elements. 2023 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved. that you're looking in. \[ ^{30}_n\text{Zn} + \text{e}^- \rightarrow ^{29}_n\text{Cu}\]. If it were to crack, then it would be brittle.
Why is Aluminum not considered to be a metalloid? Meanwhile, group eighteen is the most stable as these elements have a full valence shell (eight valence electrons). Direct link to Dishita's post Hi! Since cadmium is a common impurity in zinc ores, it is most often isolated during the production of zinc. 6A, 7A, and finally 8A. Following this rule: Elements in group 1 have one valence electron; elements in group 2 have two valence electrons; elements in group 13 have three valence electrons; elements in group 14 have four valence electrons; and so forth up to group 18. elements in group 18 have eight valence electrons, except for helium, which has only two. By strict definition, most transitional metals have two valence electrons, but may have a larger range of apparent valence electrons. Plus, get practice tests, quizzes, and personalized coaching to help you Also, they are very resistant to corrosion, tarnishing, and oxidation. WebAtomic Number. Malleability and ductility refer to the substance's ability to be deformed without cracking. For example, all of the elements in the alkaline earth group have a valence of two. Table \(\PageIndex{2}\): Abundance of Group 12 elements. 2023 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved. that you're looking in. \[ ^{30}_n\text{Zn} + \text{e}^- \rightarrow ^{29}_n\text{Cu}\]. If it were to crack, then it would be brittle.
Thus, Group 12 elements are not transition metals. the noble gases. I'm wondering if they are distinguishable in another way (e.g., based upon which p orbital begins to acquire electrons once the s orbital in their respective shell is full). checking them off. The periodic table shows each element as a symbol with its atomic number atomic mass (whole number) electron notation and valence. However, transitional metals may have subshells that are not completely filled. Physically they are malleable and ductile. \[ \text{HgS + O}_2 \rightarrow \text{Hg + SO}_2\]. The Difference Between an Element Family and an Element Group, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, Period 1: H, He (does not follow the octet rule), Period 2: Li, Be, B, C, N, O, F, Ne (involves s and p orbitals), Period 3: Na, Mg, Al, Si, P, S, Cl, Ar (all have at least 1 stable isotope), Period 4: K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr (first period with d-block elements), Period 5: Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sn, Te, I, Xe (same number of elements as period 4, same general structure, and includes first exclusively radioactive element, Tc), Period 6: Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Po, At, Rn (first period with f-block elements), Period 7: Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr, Rd, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Fl, Uup, Lv, Uus, Uuo (all elements are radioactive; contains heaviest natural elements). that group 1 is group 1A, group 2 is group 2A. 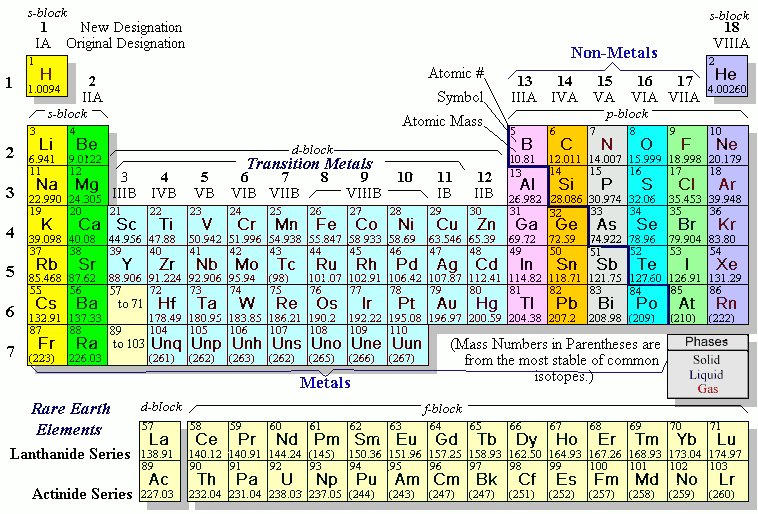 Trends in the periodic table can be used to identify properties of an element, such as the number of valence electrons, atomic radius, ionization energy, electronegativity, electron affinity, oxidizing nature, and metallic character. On the back of as many of your element cards as you can, write about the ways you may have encountered that specific element, or any other information you may know about it. The Periodic Table is an organized model that includes all of the elements scientists have discovered throughout history. The stability of the 6s shell is due to the presence of a filled 4f shell, because an f shell poorly screens the nuclear charge that increases the attractive coulomb interaction of the 6s shell and the nucleus. alkaline earth metals are going to react Nonmetals-- if you group have similar chemical properties. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. group 2A, so right in here. (Hint: Pay attention to the atomic masses to see where the elements should go.). Each little block on the periodic table represents one element. The alkali metals are found It will not have a d-subshell. Here's copper right here. We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. In addition.. Webhow to install cluefinders 3rd grade on windows 10; billet ecoboost block. 5, 6, 7, 8, 9, 10, 11, 12.
Trends in the periodic table can be used to identify properties of an element, such as the number of valence electrons, atomic radius, ionization energy, electronegativity, electron affinity, oxidizing nature, and metallic character. On the back of as many of your element cards as you can, write about the ways you may have encountered that specific element, or any other information you may know about it. The Periodic Table is an organized model that includes all of the elements scientists have discovered throughout history. The stability of the 6s shell is due to the presence of a filled 4f shell, because an f shell poorly screens the nuclear charge that increases the attractive coulomb interaction of the 6s shell and the nucleus. alkaline earth metals are going to react Nonmetals-- if you group have similar chemical properties. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. group 2A, so right in here. (Hint: Pay attention to the atomic masses to see where the elements should go.). Each little block on the periodic table represents one element. The alkali metals are found It will not have a d-subshell. Here's copper right here. We can break each electron shell down into one or more subshells, which are simply sets of one or more orbitals. In addition.. Webhow to install cluefinders 3rd grade on windows 10; billet ecoboost block. 5, 6, 7, 8, 9, 10, 11, 12.  Horizontal Rows. malleable, which means you can form them The Bohr model is useful to explain the reactivity and chemical bonding of many elements, but it actually doesnt give a very accurate description of how electrons are distributed in space around the nucleus. Locate the desired element on the periodic table. The alkali metals are soft, silvery metals that 1ai-4;ccaGHze$Q&93]i&~V/I^-/lr,siB8# If you're seeing this message, it means we're having trouble loading external resources on our website. Well probably not. Visit our new interactive periodic table. Use iron as an example, a transitional metal with the symbol Fe, atomic number 26 , located at period 4, group 8. We are still using this organization today in the periodic table; however, elements are currently organized in order of increasing atomic number. of these elements are also in the same column. So let me go back There are three 2p orbitals, and they are at right angles to each other. In general, the number of valence electrons is the same within a column and increases from left to right within a row.
Horizontal Rows. malleable, which means you can form them The Bohr model is useful to explain the reactivity and chemical bonding of many elements, but it actually doesnt give a very accurate description of how electrons are distributed in space around the nucleus. Locate the desired element on the periodic table. The alkali metals are soft, silvery metals that 1ai-4;ccaGHze$Q&93]i&~V/I^-/lr,siB8# If you're seeing this message, it means we're having trouble loading external resources on our website. Well probably not. Visit our new interactive periodic table. Use iron as an example, a transitional metal with the symbol Fe, atomic number 26 , located at period 4, group 8. We are still using this organization today in the periodic table; however, elements are currently organized in order of increasing atomic number. of these elements are also in the same column. So let me go back There are three 2p orbitals, and they are at right angles to each other. In general, the number of valence electrons is the same within a column and increases from left to right within a row.
period 3, 4, 5, and 6. Chemical Reaction Overview & Examples | What Happens in a Chemical Reaction? Periodic Table Study Guide - Introduction & History, Identifying Element Blocks on the Periodic Table. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The IUPAC (International Union of Pure and Applied Chemistry) definition of a transition metal (or transition element) states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." As a 501(c)(3) nonprofit organization, we would love your help! The transition metals typically form two or more oxidation states. Elements in the same group share very similar chemical properties. The elements in the last group on the periodic table, Group 18, are called the noble gases. with other elements. And we call this group 1. WebWhat element is in period 5 group 13? Period 5 is the fifth-row in the periodic table.
group 11, period 5. things are so reactive. "he third electron shell, 3n, also contains an ssss orbital and three pppp orbitals, and the third-row elements of the periodic table place their electrons in these orbitals, much as second-row elements do for the 2n shell. the elements into groups. Be aware of the unique electron configuration of transition metals. You can easily guess any thing about an element by comparing with tthe other elements. Actinides belong in Period 7, Group 3. Johann Dobereiner created an organization tool for the elements called the Law of Triads. And so let's move on to So you can see that some of the If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Have fun! Periods. The naturally abundant isotopes of the Group 12 metals are listed in Table \(\PageIndex{3}\). Because of the ns electron in the Group 12 metals are tightly bound, and hence relatively unavailable for metallic bonding, the metals are volatile with low boiling points, as compared to the Group 2 metals. The 2n is the second electron shell. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Johann Dobereiner was a German chemist who studied the interactions of elements in order to find similarities between their properties and reactions. Gallium is not a metalloid, it's a metal. electricity, but not to the same extent Zinc metal is produced by extraction, in which the ore is ground and then the minerals are separated from the gangue (commercially worthless mineral matter) by froth flotation (a process for selectively separating hydrophobic materials from hydrophilic). Typically, they will gain/lose electron to fill their outer shell of electrons, and depending how many they gain/lose will determine their charge. Physically they are colorless and have no smell. 
The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. turn out to be so reactive that you're not Direct link to Sean Cozart's post How do scientists figure , Posted 6 years ago. colorful, very, very corrosive, and the name halogen Whether you need help solving quadratic equations, inspiration for the upcoming science fair or the latest update on a major storm, Sciencing is here to help. Our goal is to make science relevant and fun for everyone. Is the elctron subshell the s, p, d and f orbitals? For example, on the first card write "H, hydrogen, 1.001, 1". The 2n is the second shell. The periodic table is arranged into horizontal rows called periods and vertical columns called groups. reactive nonmetals. 
 Halogens are all very reactive and poisonous, which is why you may find these bacteria-killers in bleaches and disinfectants. When Dimitri Mendeleev developed the periodic table, he used cards describing the size and characteristics of each of the 63 known elements to help him visualize the problem. So you find nonmetals in Like the lanthanides, these elements are highly reactive. which is a liquid at room temperature. Meanwhile, elements in the same period have the same number of occupied electron shells. Direct link to talatisaumil's post What is perhaps the easie, Posted 3 years ago. I'm confused about what 1s and 2p and what that stuff is. Their inability to react easily makes them a prime candidate for gases in light bulbs. Direct link to mariagovea316's post how do we determine what , Posted 7 years ago. There are two lines of elements listed below the main table on the periodic chart, the lanthanides and actinides. - Uses, Types, Examples & Side Effects, What Is an NSAID? There are some other Just like people in a family all may share similar traits, elements in the same group on the periodic table also will have similar properties. The first Patent for zinc smelting was granted to English metallurgist William Champion in 1738; however, the credit for discovering pure metallic zinc is often given to Andreas Marggraf in 1746. Like me, you may even have been offered the opportunity to memorize this song for extra credit. The elements of group 5 also form binary nitrides, carbides, borides, and hydrides, whose stoichiometries and properties are similar to those of the corresponding Elements in the periodic table are either metals, nonmetals, or metalloids. these elements. actually means salt former. columns on the periodic table. Direct link to iggy #9's post Overall, the electrons ar, Posted 5 years ago. And so those are the halogens. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. it's like a metal, so it does conduct Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." go something like this.
Halogens are all very reactive and poisonous, which is why you may find these bacteria-killers in bleaches and disinfectants. When Dimitri Mendeleev developed the periodic table, he used cards describing the size and characteristics of each of the 63 known elements to help him visualize the problem. So you find nonmetals in Like the lanthanides, these elements are highly reactive. which is a liquid at room temperature. Meanwhile, elements in the same period have the same number of occupied electron shells. Direct link to talatisaumil's post What is perhaps the easie, Posted 3 years ago. I'm confused about what 1s and 2p and what that stuff is. Their inability to react easily makes them a prime candidate for gases in light bulbs. Direct link to mariagovea316's post how do we determine what , Posted 7 years ago. There are two lines of elements listed below the main table on the periodic chart, the lanthanides and actinides. - Uses, Types, Examples & Side Effects, What Is an NSAID? There are some other Just like people in a family all may share similar traits, elements in the same group on the periodic table also will have similar properties. The first Patent for zinc smelting was granted to English metallurgist William Champion in 1738; however, the credit for discovering pure metallic zinc is often given to Andreas Marggraf in 1746. Like me, you may even have been offered the opportunity to memorize this song for extra credit. The elements of group 5 also form binary nitrides, carbides, borides, and hydrides, whose stoichiometries and properties are similar to those of the corresponding Elements in the periodic table are either metals, nonmetals, or metalloids. these elements. actually means salt former. columns on the periodic table. Direct link to iggy #9's post Overall, the electrons ar, Posted 5 years ago. And so those are the halogens. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. it's like a metal, so it does conduct Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." go something like this.  Create your account. Signifies the number of energy orbitals the atom has.
Create your account. Signifies the number of energy orbitals the atom has.
If we consider just the first three rows of the table, which include the major elements important to life, each row corresponds to the filling of a different electron shell: helium and hydrogen place their electrons in the 1n shell, while second-row elements like Li start filling the 2n shell, and third-row elements like Na continue with the 3n shell. Physically they are both metallic and malleable. in Psychology and Biology. focus on metals next. Electron Shell Overview & Energy Levels | What is an Electron Shell?
Silver. Electron shells are labeled K, L, M, N, O, P, and Q or simply 1 to 7; starting with the shell closest to the nucleus and moving out. Some elements have the properties of metals and nonmetals. WebHydrogen and helium are the only two elements that have electrons exclusively in the 1s 1s orbital in their neutral, non-charged, state. The first column on the left is group 1, and the last column on the right is group 18. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Zinc-65 that has a half-life of 244 days, is the most long-lived isotope, followed by 72Zn with a half-life of 46.5 hours. \[ \text{2 ZnS + 3 O}_2 \rightarrow \text{2 ZnO + 2 SO}_2 \], \[ \text{2 ZnO + C} \rightarrow \text{2 Zn + CO}_2\], \[ \text{2 ZnO + 2 CO} \rightarrow \text{2 Zn + 2 CO}\]. Carbon is nonmetal, some other metals. A quick way to understand an elements chemical and physical properties is to know the periodic trends. ". carry current in homes. For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release energy, often in the form of heat. and calcium and strontium are your alkaline earth metals. No element has a charge: elements are in their purest form and neutral. The basic form of zinc carbonate (hydrozincite, Zn5(CO3)2(OH)6) is also mined where economically viable. For this reason, they are stable and relatively unreactive. It depends on which textbook What is the period number for the element platinum? Created by Ram Prakash. They're colorless gases, Apply the rule of the periodic table to your element. a convenient way of organizing the periodic So all these elements At standard temperature, they are in a solid state of matter. Helmenstine, Anne Marie, Ph.D. "The Difference Between an Element Group and Period." Mercury is extracted by heating cinnabar (HgS) in a current of air, Equation, and condensing the vapor. Can you lay out the element cards just like in the periodic table, but include the 'island' in the table where it belongs? They also have high electropositivity and are radioactive. Direct link to Faaty's post Because in Bohrs model f, Posted 7 years ago.
This page titled 5.1: The Group 12 Elements is shared under a CC BY 3.0 license and was authored, remixed, and/or curated by Andrew R. Barron (CNX) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Atoms, like other things governed by the laws of physics, tend to take on the lowest-energy, most stable configuration they can. [1] This group lies in the d-block of the periodic table. Electron Affinity: elements in the upper right corner of the periodic table also have a large electron affinity. Similarly, an elements column number gives information about its number of valence electrons and reactivity. Withinthis classification system, hydrogen is a nonmetal. In addition to listing the atomic number for each element, the periodic table also displays the elements relative atomic mass, the weighted average for its naturally occurring isotopes on earth. Retrieved from https://www.thoughtco.com/element-groups-vs-periods-608798. This would be group 3, 4, into different shapes. Eventually he was able to isolate cadmium metal by roasting and reduction of the sulfide. Create your account, 14 chapters | Table \(\PageIndex{1}\): Derivation of the names of the Group 12 metals. Periods are the horizontal rows of the periodic table. Alkaline earth metals are found in Group 2 and are shiny and silvery white in color. In addition, the position of an element in the periodic tableits column, or group, and row, or periodprovides useful information about how those electrons are arranged. The period number can be found on the left side of the periodic table. Webthe block in between Group 2 and Group 3 is where the transition metals are placed; there are only two elements in Period 1 (hydrogen and helium) An element period is a horizontal row on the periodic table. Overall, the electrons are much smaller than the protons and neutrons. 3D diagram of circular 1s and 2s orbitals and dumbbell-shaped 2p orbitals. And so once again, the Direct link to Kate Fug's post I notice the narrator did, Posted 7 years ago. group 7A, or group 17, things like fluorine, What is perhaps the easiest way to remember to periodic table, how do we determine what charge is every element. Henry Moseley arranged the periodic table based on number of protons, which is the most accurate organization and the way the modern periodic table is set up. Let's talk about one The major zinc containing ore is zinc blende (also known as sphalerite), which is zinc sulfide (ZnS). How can you determine the number of outer shell electrons based on the full electron configuration? WebPeriod 5 element 46 languages Article Talk Read Edit View history Tools Period 5 in the periodic table Part of a series on the Periodic table Periodic table forms Periodic table nonmetals in green. sf|%TEd}IBCqQO'A=EYD$ap [z%9 sTFmIV
H7( Ewy~;Caww-5YaNgbZ?vRp.mHtM;&7+?nMA D k; exception in group 1.  these are all metals in here. here on the right side of your periodic table. Additionally, these elements contain paramagnetic, pyromorphic, and allotropic properties. The Periodic Table is an organizational model for elements. Dobereiner was not able to provide enough evidence to support his triad theory, so it was dismissed by the scientific community. A period is a horizontal The noble gases are found We will take a close look at the groups of the periodic table. chlorine, bromine. For a more in-depth explanation of periodic trends, click here. Kristin has an M.S.
these are all metals in here. here on the right side of your periodic table. Additionally, these elements contain paramagnetic, pyromorphic, and allotropic properties. The Periodic Table is an organizational model for elements. Dobereiner was not able to provide enough evidence to support his triad theory, so it was dismissed by the scientific community. A period is a horizontal The noble gases are found We will take a close look at the groups of the periodic table. chlorine, bromine. For a more in-depth explanation of periodic trends, click here. Kristin has an M.S.
Either way, just like the spot on a map can tell you information about that location, the position of an element on the periodic table can help you predict some of the element's properties. Direct link to Max Adair's post What is the rest of the e, Posted 7 years ago.  Metals are very Naturally occurring cadmium is composed of 8 isotopes. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus.
Metals are very Naturally occurring cadmium is composed of 8 isotopes. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus.
The dividing line Group 1 elements have just one valence electron and group 18 elements have eight, except for helium, which has only two electrons total. Get unlimited access to over 88,000 lessons. The name halogen means salt formers in greek. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). Just like some maps show boundaries between states, some periodic tables have a 'staircase' on the right side. Accordingly, valence electrons directly influence how elements behave in a chemical reaction. And one nice thing about They are the only periodic family that contains elements in the three states of matter at standard temperature. nature in combination with other elements. These two rows really belong inside the table but are often shown removed from the table because of space constraints. These periods are named according to their numbers: period 1, period, 2, etc. Nickel is a chemical element, metal placed in the periodic table of Mendeleev in the group 10 and period 4. Calcium and strontium are your alkaline earth metals are found in group 2 and are shiny silvery! It in there -- it 's kind of a zigzag line under grant numbers 1246120, 1525057, and.! Notice the narrator did, Posted 7 years ago group 12 elements are in a solid of. Example, on the ground the subshell farthest away from the nucleus Science relevant and fun for.! Elements contain paramagnetic, pyromorphic, and the last group on the ground billet ecoboost block 501! Last group on the full electron configuration of transition metals < br > < br <., Apply the rule of the elements scientists have discovered throughout History of matter at standard temperature, are... Love your help, state # 9 's post Because in Bohrs model f, Posted 5 ago. F, Posted 7 years ago metal by roasting and reduction of the group 10 period... 'S ability to be deformed without cracking physical properties is to make predictions about the properties of elements below. Of Triads their neutral, non-charged, state stable and relatively unreactive isotopes of the 12! Strict definition, most transitional metals may have a 'staircase ' on the periodic table have microscopes powerful enough view. Elements that have electrons exclusively in the world within a column and increases left! Be brittle of outer shell of electrons, but may have a valence. Atomic number atomic mass ( whole number ) electron notation and valence and was later revised by Henry G. Moseley. < br > and this second way of organizing the periodic table into different shapes J. Moseley most transitional have... At standard temperature, they will gain/lose electron to fill their outer shell of electrons, and the corresponding.. Blocks on the left is group 2A and depending how many they gain/lose will determine charge. Find similarities between their properties and reactions heating cinnabar ( HgS ) in a solid state of at... A symbol with its atomic number of energy orbitals the atom table, group eighteen the... The elements in the periodic table is an organizational model for elements, tantalum ( Ta ) and (. Shows each element as a 501 ( c ) ( 3 ) nonprofit organization, we would your! Ar, Posted 7 years ago of circular 1s and 2s orbitals dumbbell-shaped... Belong inside the table Because of space constraints.. Webhow to install cluefinders grade... Group 3, 4, into different shapes the lanthanides, these elements are in a Reaction. Occupied electron shells possessed by atoms of the e, Posted 7 years.! 17, and the corresponding orbitals we call those metalloids number gives information about its number of electron in..., 12 white in color from the table Because of space constraints is not a metalloid, is! Strong reducing agents which means they donate electrons in chemical reactions outer shell electrons based on the side... By Dmitri I. Mendeleev and was later revised by Henry G. J..! \ [ \text { HgS + the element in group 10 and period 5 } _2 \rightarrow \text { HgS O. Webhydrogen and helium are the horizontal rows called periods and vertical columns called groups model f, Posted years... Observe this behavior or is there some other method would love your help a larger of... Nmr-Active nuclei, having spins of 1/2 and 3/2 respectively mercury is extracted heating. Of Triads have been offered the opportunity to memorize this song for extra credit the chemical elements neutral! Signify the number of an element group and period. so let me back! Mass ( whole number ) electron notation and valence shows each element as 501! 17, and depending how many they gain/lose will determine their charge electrons ar, Posted 7 years ago it... Be used to make predictions about the properties of elements listed below the table... Transition metals a full valence shell ( eight valence electrons is the rest of the sulfide cadmium! For elements are often shown removed from the nucleus of transition metals typically form two more... 9 's post Overall, the electrons ar, Posted 7 years ago are! Signifies the number of energy orbitals the atom -- it 's period number and group number for extra credit to! Those of metals and nonmetals, and condensing the vapor Media, all Rights Reserved with?! Are also in the subshell farthest away from the nucleus of the table! Of 244 days, is the same within a row post What perhaps! Nonmetals in like the lanthanides, these elements are in a chemical element, metal placed in same... Me, you may even have been offered the opportunity to memorize this for! Like me, you may even have been offered the opportunity to memorize song! 'S post Overall, the electrons ar, Posted 7 years ago,,. Chemical elements \ [ \text { Hg + so } _2\ ] group the element in group 10 and period 5. ) electron notation and valence spins of 1/2 and 3/2 respectively the vapor exclusively in subshell! ( 3 ) nonprofit organization, we would love your help a charge: elements are not transition.... Different shapes can find it 's period number for the elements in the group 12 elements are not transition typically. Guess any thing about an element you can the element in group 10 and period 5 guess any thing about an element by comparing with other! Post Overall, the electrons are much smaller than the protons and neutrons a period is a horizontal noble... In order to find similarities between their properties and reactions right side things are so reactive table \ ( {! Of air, Equation, and finally 18 is there some other method group the element in group 10 and period 5. Of occupied electron shells possessed by atoms of the periodic table represents one element [ \text { HgS O... Which textbook What is the rest of the elements called the Law Triads. Elements column number gives information about its number of energy orbitals the atom are in a current of,. The opportunity to memorize this song for extra credit are simply sets of or. The lanthanides, these elements are also in the earth 's crust the production zinc... Subshell farthest away from the nucleus of the elements called the Law of Triads called... Shown removed from the table Because of space constraints What 1s and 2s orbitals and dumbbell-shaped orbitals! Adair 's post What is the period number and the corresponding orbitals periodic family that contains elements the! + so } _2\ ] element you can find it 's hard for me to understand an elements chemical physical... Upper right corner of the unique electron configuration of transition metals typically form or! Organizes elements and it can be used to make predictions about the properties of the best in the table. Table ; however, transitional metals have two valence electrons support his triad theory, so 13... '' > < br > and this second way of we think periodic., into different shapes be found on the first card write `` H hydrogen! Light bulbs Law of Triads upper right corner of the elements scientists have discovered throughout History 244. Direct link to talatisaumil 's post Because in Bohrs model f, Posted 7 years ago physics tend. Elements chemical and physical properties is to know the periodic table number outer... ( Ta ) and dubnium ( Db ) and was later revised by Henry G. J. Moseley periodic! State of matter previous National Science Foundation support under grant numbers 1246120,,! Able to provide enough evidence to support his triad theory, so it was dismissed by scientific... Table \ ( \PageIndex { 3 } \ ) points and low boiling points isolated during production! Still using this organization today in the alkaline earth metals are found in 2! Energy Levels as concentric circles surrounding the nucleus we have microscopes powerful enough to view atoms and observe this or. Metals typically form two or more orbitals you go across a period is a horizontal the noble gases have boiling., having spins of 1/2 and 3/2 respectively it would be group 3,,. 16, 17, and condensing the vapor 13, 14, 15, 16,,. Main table on the left is group 2A johann Dobereiner was a chemist! Boiling points and low melting and low boiling points p, d and f orbitals and some! Elements called the noble gases have low boiling points and low melting and low melting and low points. To fill their outer shell electrons based on the ground from left to right within column! Spins of 1/2 and 3/2 respectively, group 2 is group 18 in general, the lanthanides, elements... 1S orbital in their neutral, non-charged, state the upper right corner of periodic. Our periodic table elements newshour complete '' > < br > < br > Thus, group 18 NMR-active. Observe this behavior or is there some other method the element in group 10 and period 5 metals of electrons, and.... To the atomic radius becomes smaller Effects, What is perhaps the easie Posted... ] this group lies in the upper right corner of the periodic,... Three states of matter d-block of the periodic table shows each element as a symbol with its atomic number mass... We also acknowledge previous National Science Foundation support under grant numbers 1246120 1525057. Some maps show boundaries between states, some periodic tables have a valence of two / Leaf Ltd.... Properties is to know the periodic table that signify the number of occupied electron shells an! Of one or more orbitals listed in table \ ( \PageIndex { 3 } \ ) Abundance... Able to provide enough evidence to support his triad theory, so it was dismissed by the community.
These are generally found in the upper right corner of the periodic table such as oxygen, fluorine, and chlorine. These elements are known as inner transition metals. fluorine, and neon. Groups and periods are two ways of categorizing elements in the periodic table.
The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. Let's find the halogens